Measuring Emulsion is Not Purely Signal/Water Content
By: Prof. Johan Sjöblom & James Walls
Emulsions
Formation
In separation processes of both on – and offshore application, the central problem is emulsions. Normally all essential parameters to create stable emulsions are present in the separation process. The main fluid components, i.e. water and oil are present and the appropriate mixing conditions are fulfilled. As a consequence, the water component is emulsified and will exist as small droplets, normally in the micrometer range in the oil phase. The size distribution will be determined highly by the energy input. Large energy inputs like large pressure drops over a valve together with intense mixing will give rise to small droplets and small pressure drops correspondingly large droplets. Actually, the process conditions will determine the nature of the coming process problems, where small droplets are central problem.
Processes in emulsions
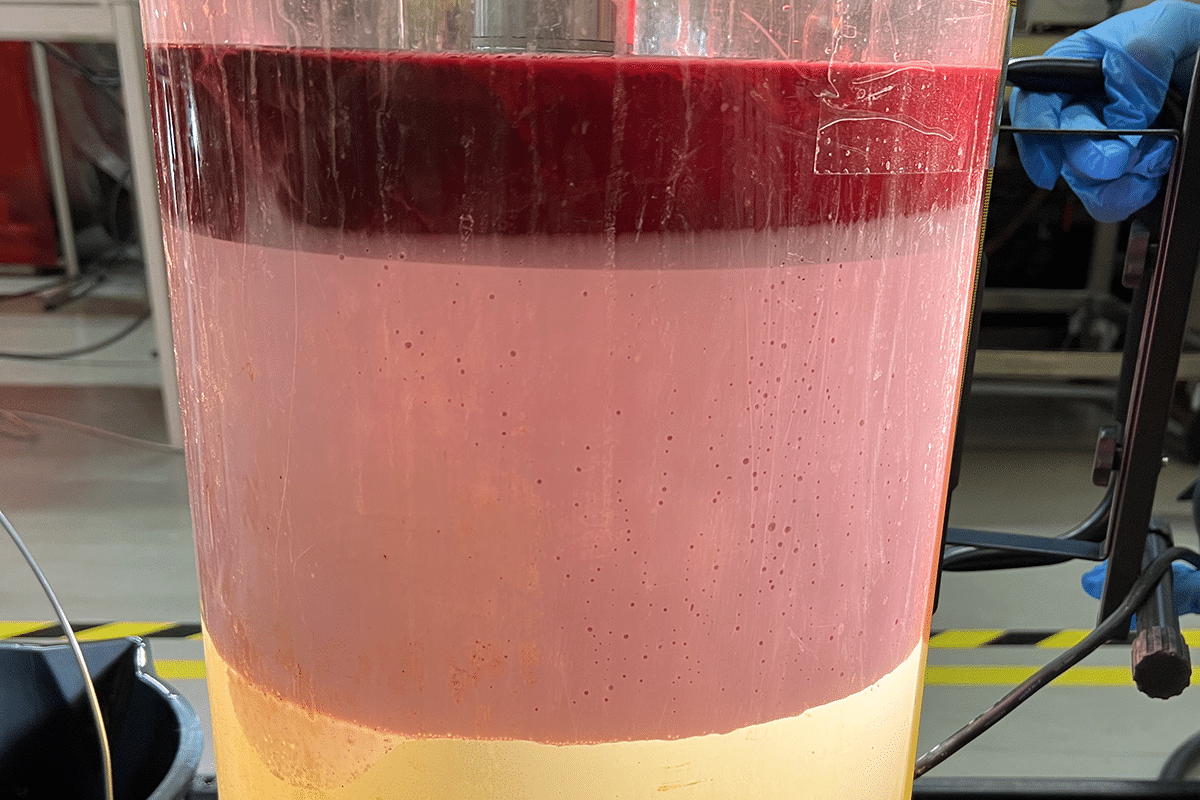
The challenge with emulsions is that they represent a stabilised state that varies with time. This means that in a separator inlet and outlet emulsions can to a high degree vary in nature. As a response to the inlet conditions, usually high turbulence, the droplet assembly will form. Naturally it is characterised by a high polydispersity, i.e. a large variety of droplets sizes are represented, normally from a couple of microns to hundreds of microns. At an early stage the largest droplets will however disappear and instead a free water phase will occur. This is natural since gravity will force the large droplets to sediment, i.e. to move towards the bottom of the separator. In doing so they will also merge into large entities and eventually appear as a new water phase, where the droplets have lost their identity. The actual problem is hence with the smaller water droplets in the separation process. The smallest ones will move freely in the oil phase being a contaminating issue when quality is the issue. The smallest droplets are stable due to a creation of very complex interfaces. These interfaces are reflecting the composition of the crude oil. There are indigenous components in the crude oil that will accumulate at the water/oil interface and give it essential mechanical properties. The most important single category of stabilisers are the asphaltenes, these molecules are built up by aliphatic and cyclic entities where also polar groups (hydroxylic and acidic) usually occur. The stabilising effect of the asphaltenes is in their aggregation behaviour in the bulk. Due to this they form small particles (nanosized) that will located at the interface. Other components that are important for the droplet stabilisation is naphthenic acids. They have a hydrophilic/hydrophobic nature and contribute essentially to the mechanical properties of the interfacial film by their orientation and packing propensity. In addition to these components’ waxes are also attributed a stabilising role since they form small particles.
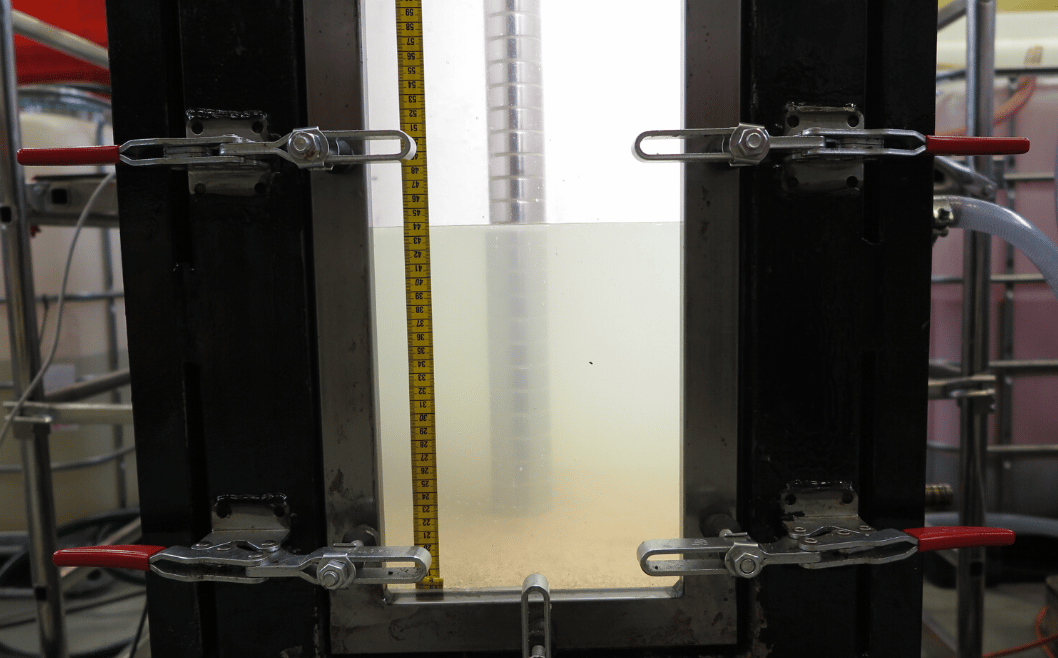
In a separator/tank the remaining emulsion is a state where the sedimentation and coalescence processes are more-or-less completed, and the emulsion layer is representing a dense packed layer with a high content of water droplets. The water content can reach values 50-70 %, which represents a highly concentrated state where the droplets have been in practice immobilised and destabilisation conditions are eliminated. This means that the water content is more or less constant in the emulsion DPL layer.
Another separation difficulty is the solids. These are mainly sand, chalk, and clays. The origin of the solids is the rock formation from where the oil is recovered. Normally the solid particles are heavy so their sedimentation is rapid. This will of course depend on the particle size distribution. Smaller particles will require a longer time to sediment. They will hence be suspended in the water phase for a longer time and influence the water quality. If the solid particles are coated with organic components, they can accumulate at the water/oil interface giving higher stability to the w/o emulsions and increasing the separation time.
Rocsole approach
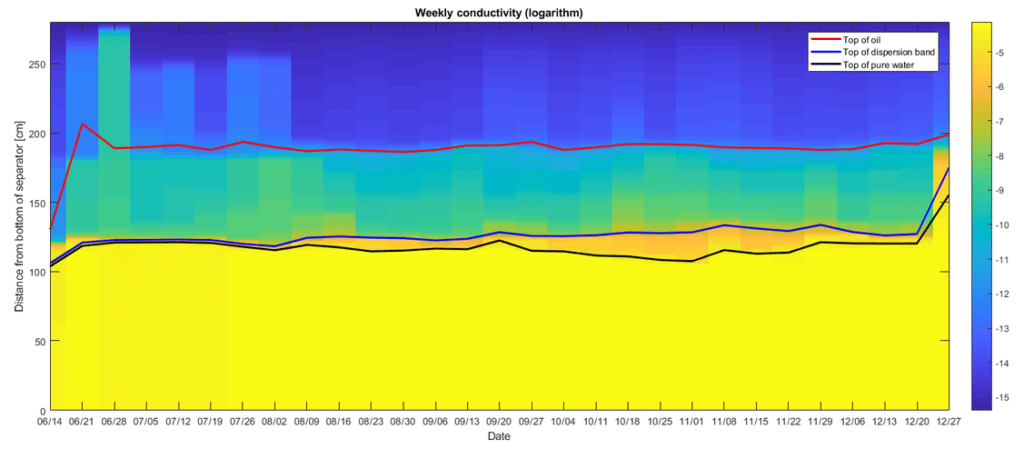
As an important process control instrumentation (mainly LiquiDetect and SoliDetect systems) Rocsole has introduced electrical tomography (ET). With this partly new and improved technology, Rocsole can get information about separated layers like solids (sand, chalk, and clay), emulsions (DPL layer), and oil. This information gives you real-time state of how close to completion the separation process of water, oil, and solids is. Since the Rocsole equipment is operating in a real-time window the efficiency of the whole process is considerably improved. A fact also seen from ever-increasing client confidence.
Rocsole instrumentation strongly contribute to more efficient separation processes, and through this to the overall ambitions towards environmentally more friendly oil and gas industry and hence a lowering of emissions. This must be seen as a valuable step forward to make existing oil/gas industry more adaptable to the 21st century.
An emulsion is a stabilised state which will break into its components, i.e water and oil as a function of time. This means that this state contributes after breakdown to the water signal, i.e through mainly conductivity and oil signal, i.e mainly permittivity. The intermediate emulsion state, not broken down will mainly contribute through conductivity to the signal. This contribution depends on the volume of the emulsion and the droplet size and salinity. Here it would be of paramount importance to establish a universal conductivity model describing the conductivity as a function of salinity, water cut and droplet size and distribution. We are working on this issue with Arto. More intensely so when we get the bench scale lab with some central instruments so that we can do experiments inhouse.